X Acquire product or service sheets Already a customer of Demaco Holland B.V.? You have already got use of the connected files but Be at liberty to submit a whole new ask for.
Biopharmaceutical companies have significantly favoured lyophilization with the formulation in their pharmaceutical products. Mostly, the driving components leading to the enhanced utilization of lyophilization is the opportunity to stabilize the drug product or service and excipients in the reliable matrix, increasing the shelf life of the solution. This, combined with the removing of solvents, incorporates a good influence on storage and distribution prerequisites.
The chamber is sterilized in a temperature of 121°C utilizing a entirely validated, Pc-controlled automated steaming method
Controlled freezing premiums, in conjunction with annealing and process familiarity with supercooling consequences, are sometimes utilized to attain uniform ice crystal distribution. More recent systems are delivering the opportunity to nucleate on demand from customers, even further growing product uniformity throughout lyophilizer shelves, and is also a spotlight in long term lyophilization know-how.
Contemplate All those lightweight, but flavor-packed freeze-dried berries with your cereal or the moment coffee that wakes you up each morning.
Backbone BioPharma is only one of many biopharmaceutical companies in search of assistance inside the lyophilization process, which is developing in reputation.
We are uniquely positioned to establish lyophilization cycles from the beginning or to enhance present cycles, offering a commercially desirable however affordable process. Owning created in excess of 675 lyophilization cycles, shopper companions rely on us to achieve their Good quality Target Product Profile and supply lifestyle-modifying therapies to sufferers.
Lyophilization includes a series of ways to realize exceptional merchandise stability and high-quality. When there are particular person intricacies within these methods, they may be broadly classified into 3 phases: freezing, check here Major drying, and secondary drying.
Purposeful cookies help to carry out specific functionalities like sharing the written content of the website on social websites platforms, collect feedbacks, together with other 3rd-party capabilities. Performance Functionality
Lyophilization is usually a process that will involve freezing a liquid drug product or service after which removing the frozen solvent through sublimation, delivering a steady reliable matrix of drug product or service and also other excipients.
The next move inside the process is secondary drying. Secondary drying takes what is a lyophilization process place when the last ice crystal has disappeared, as well as merchandise is then cautiously warmed up from its minimal temperature. This closing dehydration of your item is completed below a higher-temperature vacuum that rids the procedure of any water that didn't crystallize and was certain to the item’s molecules.
Our TFF techniques are adaptable for process advancement and clinical trials in laboratory environments together with for industrial production batches.
During the lyophilization process for pharmaceutical manufacturing, there are actually three broad stages: freezing the solution, setting up a vacuum, drying the solution below vacuum at an exceptionally minimal temperature.
Linda understands what functions to look for when buying or replacing your laboratory's freeze dryer. Find out far more On this new Lab Manager online video.
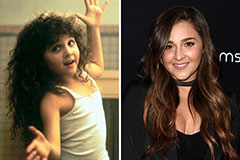 Alisan Porter Then & Now!
Alisan Porter Then & Now! Charlie Korsmo Then & Now!
Charlie Korsmo Then & Now! Shane West Then & Now!
Shane West Then & Now! Brooke Shields Then & Now!
Brooke Shields Then & Now! Megyn Kelly Then & Now!
Megyn Kelly Then & Now!